Horizontal Guided Bone Regeneration of the Posterior Mandible to Allow Implant Placement: 1-Year Prospective Study Results
Jonas Lorenz, Shahram Ghanaati, Marko Magić, Bastian Wessing, Giorgia Mariotti, Eriberto Bressan, Zoran Aleksic, Iva Milinkovic, Zoran Lazic, Ramona Schleich Grotenclos, Mauro Merli, Luca De Stavola, Robert Sader
Abstract
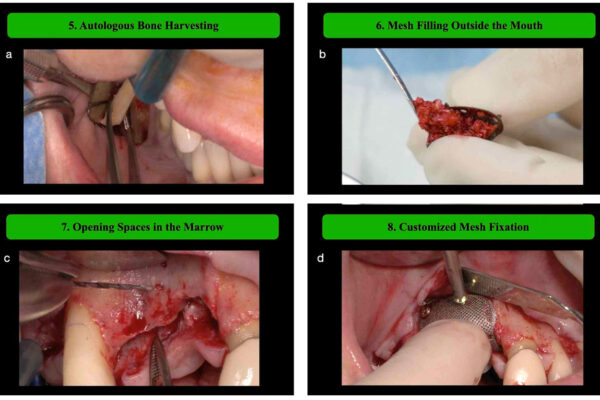
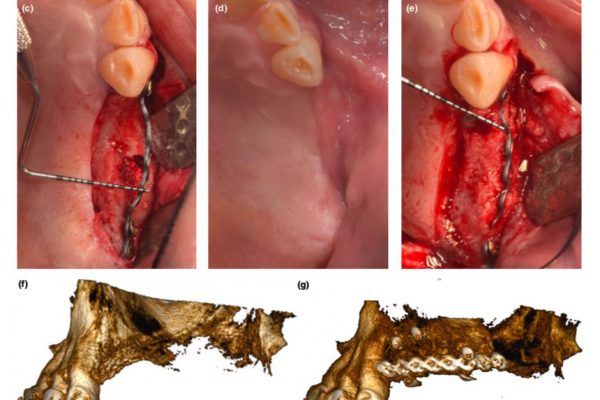
Objective: Assess whether horizontal ridge augmentation with guided bone regeneration (GBR) using deproteinized bovine bone mineral (DBBM), autologous bone, and a resorbable collagen membrane supports successful implant placement. Materials and Methods: This open, prospective, single-cohort, multicenter clinical study included patients with ridge defects that required GBR prior to implant insertion. The primary endpoint was radiologically assessed bone gain after 8 months post- GBR, measured at the center of planned implant sites. Secondary endpoints included implant survival and success, marginal bone levels (MBLs), MBL changes, and soft tissue health.
Results: Of 45 patients evaluated 8 months post-GBR, nine experienced dehiscence in the first 3weeks of the healing period. GBR led to radiologically determined mean bone width gain of 4.0±1.5mm and 4.8±1.7mm, measured 1 and 3mm from the top of the crest, respectively, allowing successful implant placement in 44 patients (97.8%). The cumulative implant survival and success rates were 98.9% and 95.5%, respectively. MBLs were stable: −1.18±0.64mm at definitive prosthesis placement (DPP) and − 1.07±0.74mm at 1 year. Soft tissue health and esthetics (plaque and bleeding indices, papilla, keratinized mucosa, and pink esthetic score) improved from DPP to 1 year. Patients were highly satisfied with implant function and esthetics, and their oral health-related quality of life improved.
Conclusions: GBR using DBBM and a collagen membrane offered a safe and effective treatment option for horizontal ridge augmentation sufficient to support implant-based tooth rehabilitation.
Trial Registration: Registered at ClinicalTrials.gov NCT03028922 (registrations sites, as above listed affiliations, first posted January 23, 2017)
Clinical Oral Implants Research, 2024; 0:1–17